
Source: Michigan State University Extension, Angel Abuelo, Arpita Nayak, Katy Kesler, Hannah Carlson and Faith Cullens-Nobis
Dairy cattle can succumb to illnesses at any time but most diseases take place around two clusters: the time around calving and the first few weeks of a calf’s life. A key factor responsible for the development of these diseases is oxidative stress.
Oxidative stress results from the imbalance between the production of free radicals, also known as reactive oxygen/nitrogen species (RONS), and the availability of antioxidant defenses that are needed to reduce RONS-induced cellular damage.
Studies performed in the last decade clearly indicate that dairy cows experience OS around the time of calving and the first few weeks of age. OS diminishes functional capabilities of immune cells and increases the animals’ susceptibility to diseases. In this article, we will review some of the work our group has been doing on characterizing OS in transition cows and newborn calves, as well as strategies to mitigate its impact through antioxidant supplementation.
Oxidants and antioxidants
Oxidants are substances that are able to oxidize other molecules. Free radicals are oxidizing agents with one or more unpaired electrons in the outer electron shell, making them highly reactive. RONS are the most abundant free radicals in biological systems, and are formed normally as byproducts of cellular metabolism. RONS are essential for cell physiological processes, and play a key role in production of cell signals and the destruction of pathogens by the immune system. When produced in excess, however, RONS can harm the cells, leading to loss of cell function and tissue damage.
To prevent impaired biological function due to RONS damage to cellular structures, living organisms have developed a complex antioxidant defense system. Antioxidants can be divided into three major groups: enzymatic antioxidants, nonenzymatic protein antioxidants and nonenzymatic low-molecular-weight antioxidants. Of these, the nonenzymatic antioxidants are primarily responsible for the antioxidant capacity of plasma. For example, lipid-soluble α-tocopherol (vitamin E) protects cell membranes from lipid peroxidation; ascorbic acid (vitamin C) and β-carotene can neutralize free radicals and enhance the antioxidative effect of vitamin E. Similarly, several trace elements, such as copper, manganese, selenium and zinc can protect the body from RONS either directly or as cofactors of antioxidant enzymes.
Oxidative stress vs. oxidant status
These terms have been used interchangeably in the past. However, we now know that there is a clear difference between OS and oxidant status that should be considered. Oxidant status refers to the balance between the production of RONS and the total antioxidant capacity, also known as reduction-oxidation (redox) balance. Conversely, OS refers to the oxidative damage resulting from the imbalance between oxidants and antioxidants. OS includes oxidative modification of cellular macromolecules, cell death, as well as structural tissue damage (Figure 1).
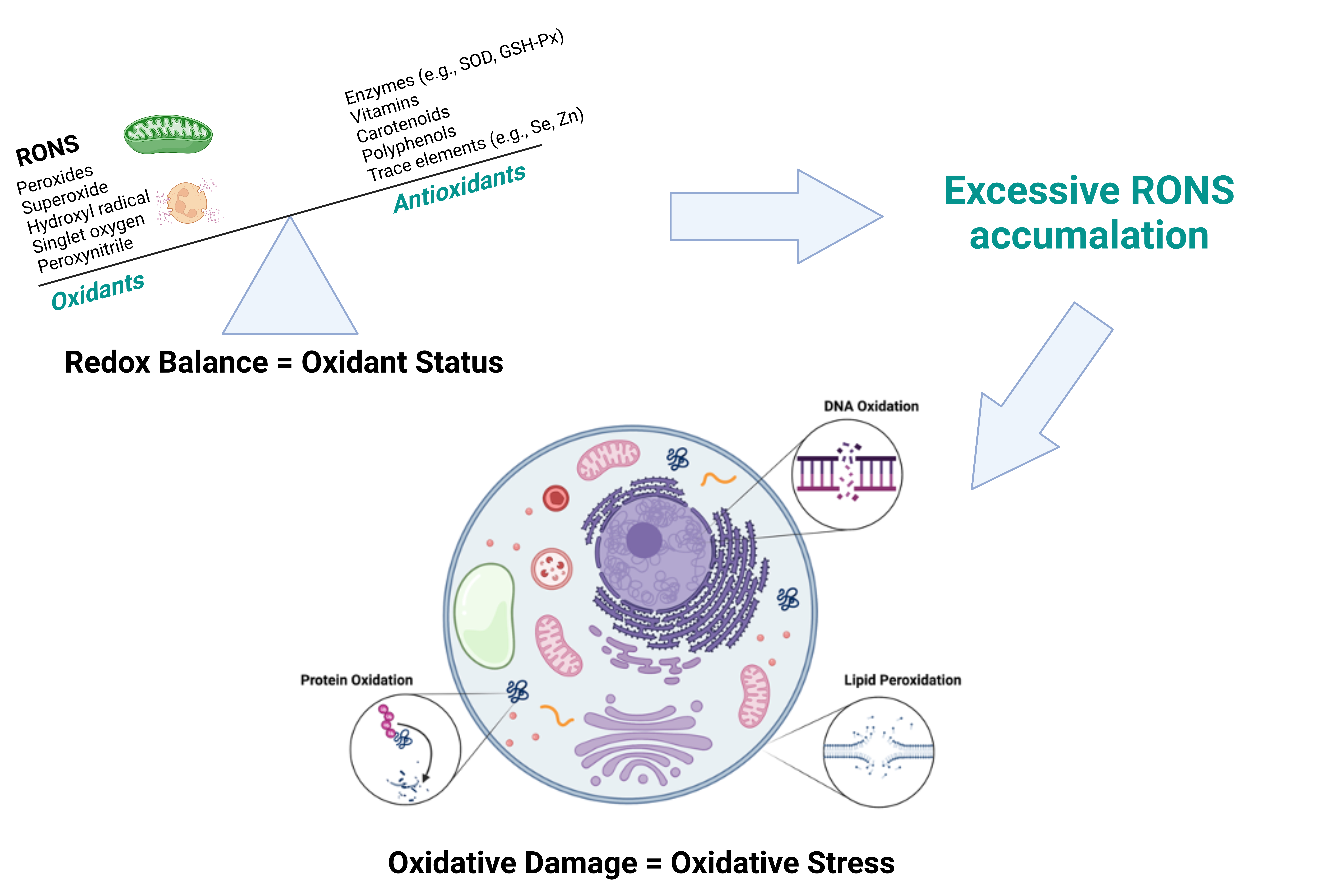
It is expected that oxidative damage occurs as a result of shifts in the oxidant balance. Nevertheless, not all shifts in redox balance will result in OS. RONS are essential for many physiological processes such as cell growth and proliferation. Therefore, changes in oxidant status might just reflect changes in redox signaling that are not associated with cell or tissue dysfunction.
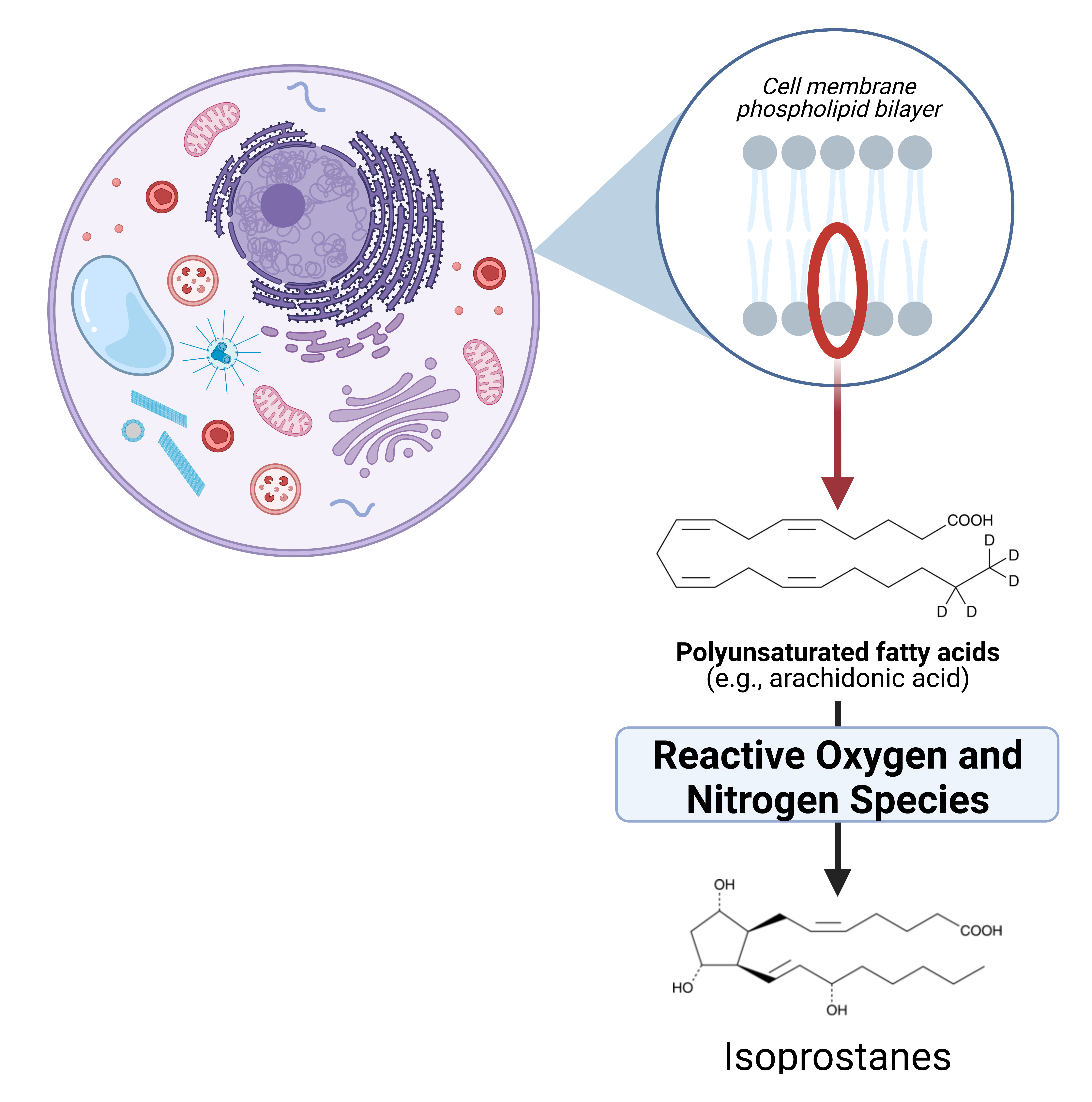
This difference between OS and oxidant status also impacts the information that different biomarkers provide. In our group, we have validated the Oxidant Status index (OSi; calculated as the ratio between RONS and total antioxidant capacity) to characterize redox status in biological fluids, but measure oxidized macromolecules to evaluate OS. For example, we routinely quantify isoprostanes, a family of prostaglandin-like compounds formed through the oxidation of fatty acids in cellular membranes (Figure 2), and advanced oxidized protein products to evaluate the oxidative damage to cellular lipids and proteins, respectively.
Oxidative stress and transition health
Dairy cows go through dramatic physiological changes to prepare for lactation. In the transition cows, dry matter intake decreases, whereas energy demands for lactation increase, resulting in energy deficit. To meet the increased energy demands, cows release body reserves, predominantly from adipose tissue. Increased fat mobilization because of energy deficit increases the generation RONS. An increase in RONS production coupled with the decreased intake of dietary antioxidants due to decreased feed intake can lead to a pro-oxidant shift that ultimately results in OS.
Oxidative stress is a significant factor underlying the dysfunctional host immune and inflammatory responses that can increase the susceptibility of transition cows to health disorders. OS is known to diminish the function of immune cells such as phagocytes or lymphocytes, and therefore increase the animals’ susceptibility to infectious diseases. Also, RONS can activate inflammatory responses in cells such as macrophages or dendritic cells. Thus, OS is also involved in the dysregulated inflammatory responses typically seen in fresh cows.
Moreover, OS during the dry period can also have effects on the offspring. Our results showed that calves born to cows that underwent greater OS during late gestation showed less robust innate immune responses during the first month of life (Ling et al. DOI: 10.3168/jds.2017-14038). Similarly, we recently showed that the antioxidant capacity of cows during the last months of gestation might be a limiting factor in the volume of colostrum produced (Rossi et al., DOI: 10.3168/jds.2022-22240). Supplementation trials are currently underway to further evaluate these associations.
Biomarkers of oxidative stress for prediction of disease
Biomarkers of OS and oxidant status have been proposed as potential predictors of transition cow disease. Nevertheless, neither reference intervals nor cut-off points for OS biomarkers have yet been established to identify individual cows suffering from OS or to predict the likelihood of disease events or impairment of production outcomes at the herd level. Therefore, the application of these biomarkers in the field is still limited.
Nevertheless, a recent study showed that biomarkers of oxidant status had a greater ability to predict fresh cow diseases at dry-off compared to common metabolic biomarkers such as non-esterified fatty acids (NEFA), beta-hydroxybutyrate (BHB), and calcium. Thus, including biomarkers of OS in herd monitoring protocols has the potential for allowing earlier detection of cows/cohorts at risk and to better inform nutritional management strategies such as antioxidant supplementation.
With a competitive grant from the USDA, our team is currently investigating the accuracy of markers of OS to predict cows at risk of developing fresh diseases early in the dry period. Current blood biomarkers used on farms for herd monitoring (e.g., NEFA or BHB) are only predictive of disease risk when analyzed during a few days around calving, precluding their use to implement corrective strategies to prevent early lactation diseases in the affected group. We aim to establish, for the first time, critical cut-off points for markers of OS in dairy cattle to allow for dry period monitoring protocols so that cows at risk of developing transition diseases can be identified with sufficient time to implement corrective measures.
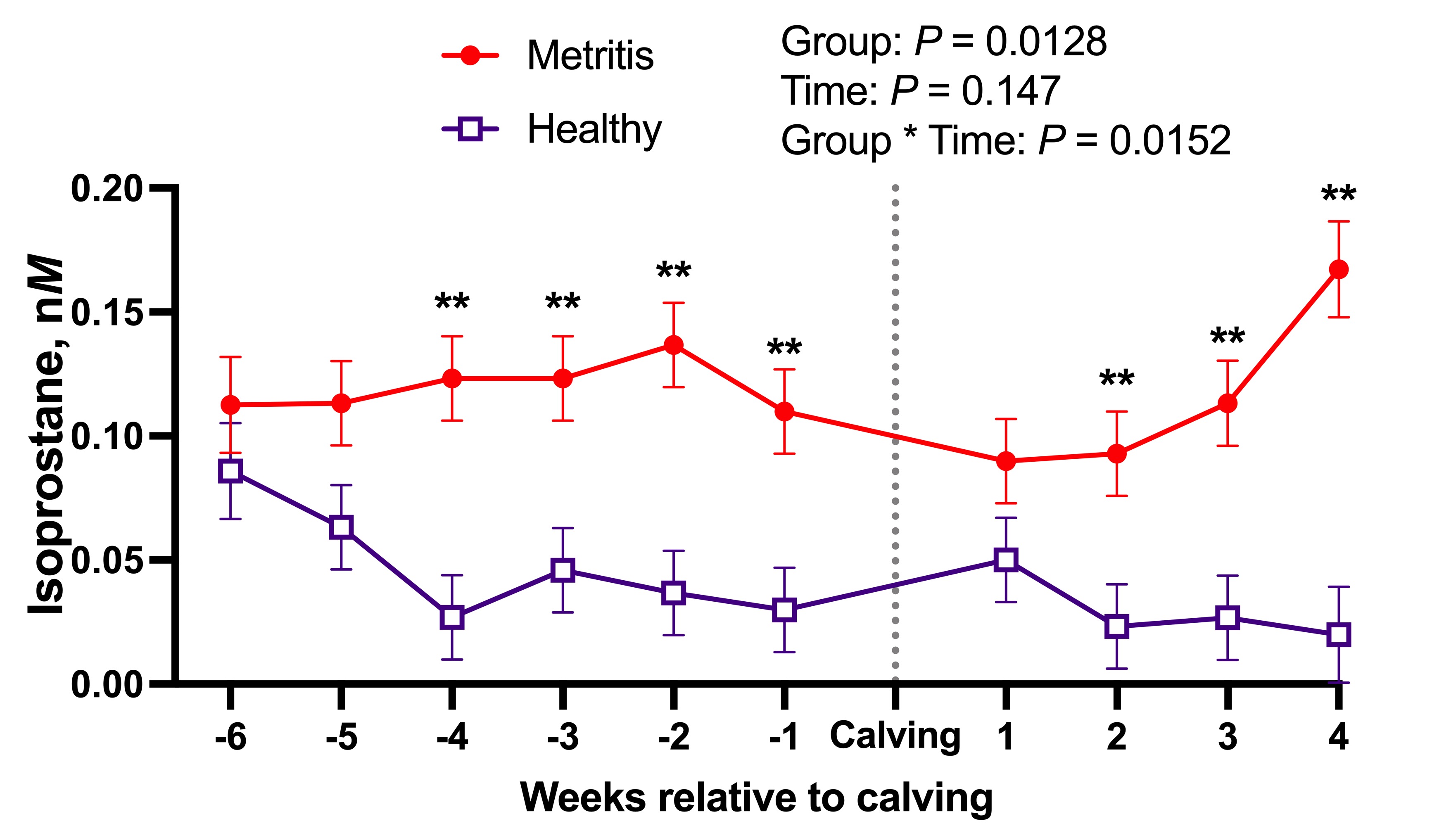
Our preliminary data shows promising results. The concentration of one isoprostane was increased up to 4 weeks before calving in cows that experienced metritis in early lactation compared to those that exhibited a healthy transition (Figure 3). As part of this project, we will also investigate which nutritional interventions could be implemented during the close-up phase to ameliorate OS in at-risk cows and avoid the development of fresh cow problems.
Oxidant status and pre-weaning diseases
After birth, calves are exposed for the first time to an oxygen-rich environment when they start to breathe, resulting in an increase in RONS production. RONS concentrations in the blood of calves was 30% higher than in their dams shortly after birth and before colostrum ingestion. Our own results also suggest that calves are experience a greater redox imbalance than periparturient cattle. Hence, OS might play a very significant role in neonatal calf health; indeed, OS has been associated with important calf diseases such as diarrhea and pneumonia.
Another critical factor to consider is the role of OS in the modulation of the immune response of newborn calves. In line with research in humans, the oxidant status experienced by newborn calves was associated with differences in their profile of cytokines (signals produced by immune cells). We further evaluated effects of in vitro OS on neonatal calf lymphocytes, the immune cells responsible for vaccination success. Our results showed that OS reduces the capacity of these immune cells to activate upon stimulation, to synthetize antibodies, and to release cytokines to communicate with other immune cells (Cuervo et al., DOI: 10.3390/antiox10020255). These are all critical functions to fight a pathogen or respond to a vaccine. Thus, OS during the pre-weaning period is a contributing factor to the dysfunctional immune responses exhibited by young calves.
Antioxidants and calf health
As for transition cows, several strategies exist to decrease the risk of OS in neonatal calves.
Maternal supplementation:
In humans, maternal supplementation of antioxidant vitamins and minerals during gestation has long been recommended to reduce OS at delivery. Some studies in cattle have also shown that dry-period antioxidant supplementation enhances the antioxidant profile of newborn calves. Nevertheless, various factors limit this delivery route in cattle: (1) the nature of the ruminant placenta limits the types of antioxidants that can be transmitted to the fetus, (2) dry dairy cattle are usually already supplemented with considerable amounts of some antioxidants (e.g., selenium close to the US legal limit of 0.3 ppm) for the prevention of transition diseases, and (3) excessive antioxidant supplementation can have downstream effects in the health of dairy cattle and has been linked with stillbirths in humans. Hence, supplementation of dry cows to increase calves’ antioxidant pool might not be an effective strategy.
Supplementation of colostrum:
In addition to immunoglobulins, colostrum is also rich in other beneficial substances such as immune cells, growth factors, cytokines, etc. Given that colostrum is the first meal that a calf should receive shortly after birth, its antioxidant content is important to offset the birth-associated OS. However, compared to normal milk, colostrum has the same amount of oxidants but less antioxidants, with the concentration of the latter increasing progressively from the first colostrum onwards. Hence, colostrum provides antioxidants to calves but is also a source of pro-oxidants. Nevertheless, newborn calves seem to be able to effectively counter the birth-associated OS, with calves showing a gradual decrease in oxidant status biomarkers. To the best of our knowledge, however, no study has compared the redox balance between calves that ingest colostrum shortly after birth with those experiencing delayed colostrum ingestion. Hence, it remains unexplored whether this gradual decline in OS following birth is due to the transfer of antioxidants via colostrum, the activation of antioxidative pathways in the calves, or a combination of both.
In addition, colostrum’s redox balance seems to play a role in immunoglobulin absorption. Selenium supplementation of colostrum increases immunoglobulin absorption, and the colostrum redox profile was significantly associated with calves’ serum immunoglobulin concentrations. However, none of these studies demonstrated which mechanisms might be implicated and therefore further research is needed. Some research at MSU has also investigated the impact of supplementing colostrum with antioxidants and fatty acids. However, although the initial results showed a positive effect on calf redox status, those effects were not reflective of improvement on calf health.
Supplementation of calves:
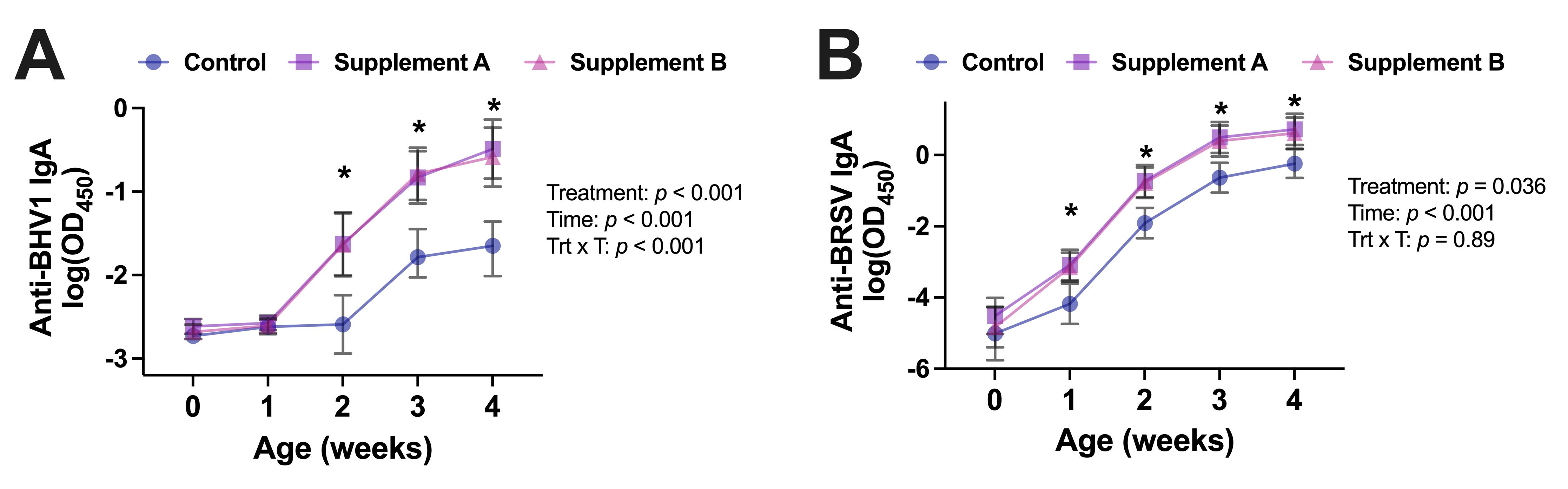
Other ways of increasing the antioxidant potential of calves are the parenteral or dietary administration of vitamins and trace elements. This is a routine management practice in many farms within the first days of life. It’s been well-established that vitamin supplementation of dairy calves can increase their performance, metabolism, and immune system. Some studies have shown a reduction in pre-weaning disease incidence, which some attribute to the antioxidant’s enhancement of immune function. Parenteral trace mineral supplementation (zinc, selenium, manganese, and copper) at 3 and 30 days of life resulted in increased the function of neutrophils (immune cells). Our team recently demonstrated that administration of parenteral antioxidant supplements simultaneously with an intranasal viral vaccine to newborn calves resulted in an increased response to the vaccine as assessed by antigen-specific immunoglobulin A antibody concentrations in nasal secretions throughout the first month of life (Figure 4; Nayak et al., DOI: 10.3390/antiox10121979).
Cautions around antioxidant supplementation
Vitamins and certain trace minerals have proven effective at counteracting OS and the severity of diseases such as mastitis or metritis, both through direct antioxidant effects and by enhancing the immune response. Most of the established nutritional requirements traditionally focus on preventing deficiency situations and these requirements are different than those for optimal immune function. Indeed, there is now evidence that supplementation slightly above reported requirements can improve animal health status and performance, as well as milk and meat quality.
On the other hand, some studies reported negative effects of excessive antioxidant supplementation, such as increased cases of mastitis or even toxicosis in calves. To date, it has been unclear at what level antioxidant supplementation stops being beneficial and starts to be associated with harmful consequences. Hence, antioxidant supplementation strategies must be implemented only to levels slightly above current recommendations unless strong scientific evidence is available to support its inclusion at a higher rate. Our team is currently undertaking a field trial to investigate the dynamics of antioxidants supplemented to calves to provide evidence-based recommendations to producers.
Redox balance is essential for several biological processes of dairy cows and calves. However, when an imbalance exists between the production of pro-oxidants and the animals’ antioxidant abilities, OS can develop, and this has been associated with immune and metabolic dysfunction. However, antioxidant therapy can protect against OS conditions, and several methods for delivery of antioxidants are routinely used in dairy farms. Antioxidant supplementation levels for optimized immune function are usually above the nutritional requirements established through traditional methods. However, excessive antioxidant supplementation can negatively impact animal health. Thus, a better understanding of the regulation of antioxidant networks and the establishment of critical cut-offs for concentration of OS biomarkers are needed to be able to provide evidence-based guidance on levels and timing of supplementation that provide an effective improvement of the animals’ health status.








