
Source: University of Georgia Extension
Introduction
Antibiotic therapy continues to play an important role in the control of mastitis in dairy cows. Lactational therapy is effective against Streptococcus agalactiae but less successful against infections caused by Staphylococcus aureus and other causes of mastitis. As a result, alternative treatment strategies have been developed, including a combination of both intramammary infusion and the parenteral administration (injection) of antibiotics to successfully cure quarters infected with S. aureus. Likewise, extended therapy, which involves prolonged drug administration, has improved cure rates against this organism. Nonantibiotic approaches to treatment have included oxytocin injections, but relapse rates after this form of therapy can be unacceptably high. Dry or nonlactating cow therapy is almost always more successful than lactational therapy because cure rates are higher and new cases of mastitis are prevented. To reduce antibiotic usage, selective dry cow therapy is becoming popular, and teat seals are appealing because they prevent new infections without having to rely on antibiotics.
Eliminating cases of mastitis
The basic principle of mastitis control is to prevent new infections, but inevitably, new cases of mastitis will occur. Once infection is established in the udder, there are four ways to eliminate the disease: spontaneous cure, the culling of chronically infected cows, treatment during lactation, and dry cow therapy. Among these four means of managing infections, antibiotic treatment is the principal method for eliminating cases of mastitis and is the primary reason for using antibiotics in dairy cows.
Successful antibiotic therapy involves drugs reaching all sites of infection within the infected quarter, remaining at adequate levels at all sites of infection for an adequate period of time, and killing all infecting microorganisms.
Standard goals of antimicrobial therapy should include:
- Enhancing animal well-being;
- Returning the cow to normal milk production and composition;
- Preventing mortality in peracute cases;
- Eliminating infectious microorganisms;
- Preventing new infections, especially in the dry period;
- Avoiding drug residues in milk and meat;
- Keeping existing cases from becoming worse;
- Minimizing damage to secretory tissues;
- Reducing spread of existing infections to other cows; and
- Improving the overall health of the herd.
Spontaneous recovery
“Spontaneous recovery” is the term used when the cow’s immune system becomes engaged and eliminates an infection without the use of antibiotics. Research has shown that this occurs in only about 20% of confirmed infections for a variety of mastitis-causing bacteria. Most spontaneous recoveries occur in quarters with mild or recently acquired infections, but only rarely in the case of well-established or chronic infections such as S. aureus. Soon after an infection is established in the mammary gland, changes in the cow’s immune system take place in an attempt to eliminate the infecting microorganisms. For example, greater leukocyte or white blood cell (somatic cell) numbers move from the blood into milk and their killing ability is increased. With the help of antibodies from the bloodstream, as well as those locally produced in the udder, these leukocytes function to overcome the infection by engulfing and killing the infecting microorganisms. Spontaneous recovery from specific infections may be enhanced in cows that have been vaccinated against the specific bacteria, such as S. aureus and E. coli, because the process of vaccination increases antibody production against specific bacteria.
Culling chronically-infected refractory cows
Culling is often the only practical means to eliminate chronic infections that do not respond to repeated attempts at therapy. Research has shown that only 7% of cows are responsible for about 40% of all clinical mastitis. Other studies have shown that 50% of all discarded milk comes from only 6% of the cows. In addition, research studies have revealed that 64% of cows that have had two cases of mastitis in the current lactation will have another clinical episode before the end of that lactation. This figure increases to 70% for cows that have already had three clinical cases. Moreover, older cows have more cases of clinical mastitis than younger cows. Such chronically infected cows exhibiting periodic flare-ups of clinical mastitis are probably infected with contagious pathogens, such as S. aureus, Strep. agalactiae, or Mycoplasma spp. These animals constitute a reservoir of microorganisms that may ultimately spread to uninfected cows, and should be culled from the herd.
Treatment during lactation
It is obvious that both spontaneous recovery and culling have serious limitations in terms of usefulness for eliminating udder infections in the herd. This leaves antibiotic therapy (lactation and dry cow therapy) as the principle alternative for eliminating existing infections. By eliminating infections, it is possible to reduce the herd level of mastitis in months rather than years. When sulfonamides and antibiotics were first introduced during the 1930s and 1940s, there was hope that these drugs would soon put an end to mastitis. Initial optimism faded when it became evident that many chronic infections could not be cured. In addition, some microorganisms demonstrated the ability to develop drug resistance. Antibiotics do, however, continue to play an important role in mastitis control. They are useful in curing many cows of existing infections and can save the lives of some animals.
The primary concern of most dairy farmers is how to make the best use of antibiotics and other drugs in treating clinical cases during lactation. Such cases require prompt and appropriate attention, though each case must be considered on an individual basis. In general, treatment in lactation is indicated when 1) cows are infected with Strep. agalactiae; 2) the herd somatic cell count (SCC) is above 200,000/milliliters (ml); and 3) when clinical mastitis is present. In most studies, the average incidence of clinical mastitis is around 20-25 cases per 100 cows per year, whereas prevalence of subclinical mastitis among cows ranges from 12.6-30%.
Therapy of acute clinical (toxic) mastitis: This infrequent form of infection is characterized by a sudden onset of disease including local symptoms such as redness, swelling, and hardness of the affected quarter, which is also sensitive to the touch. Milk appears grossly abnormal (purulent, serum-like, watery, bloody, with or without flakes and clots) and milk production is suddenly and drastically reduced. Such infections are most frequently caused by coliform bacteria such as E. coli and Klebsiella pneumonia. When growing in the mammary gland, these bacteria produce a poison or toxin called “endotoxin,” which results in systemic symptoms including increased rectal temperature, loss of appetite, recumbency, reduced rumen function, increased pulse rate, severe depression, progressive dehydration, inability to stand, diarrhea, shivering, and weakness, all of which vary with individual cases. These conditions are collectively referred to as “toxemia.” To be successful in alleviating distress, reducing pain, and improving animal well-being, therapy must be directed primarily against endotoxin, which eventually reaches the bloodstream and causes the above symptoms as well as shock.
Because most treatment regimens for acute clinical mastitis require the extra-label use of drugs, practitioners, with an established veterinary-client-patient relationship, are often called upon to formulate therapeutic management programs. Many of these programs involve the frequent stripping of affected quarters, systemic administration of carefully selected antibiotics, administration of electrolyte fluids, anti-inflammatory agents, glucose, bicarbonate, and calcium. Careful administration of calcium solutions may be undertaken if serum biochemistry values warrant such therapeutic measures. These cows are very susceptible to cardiac arrhythmias, cardiac failure, and death if calcium is administered too rapidly or is not indicated.
Although substantial numbers of coliform isolates are usually sensitive in laboratory tests to various antibiotics including cephalothin, tetracycline, ampicillin, erythromycin, and sulfonamides, the therapeutic value of these antibiotics against coliform bacteria is highly questionable. Moreover, promoting the rapid killing of coliform bacteria with the use of antibiotics may actually enhance release of the endotoxin, resulting in an animal that succumbs more quickly to endotoxin-induced shock. Most veterinarians are now placing an increased emphasis on supportive therapy to counteract endotoxin-induced shock. Severely affected cows may need 40 to 60 liters(quarts) of fluids intravenously in the first 24 hours after onset of the disease. Selected anti-inflammatory drugs are often used to counteract the effects of the endotoxin. Systemic antibiotics are often considered adjunctive to supportive therapies such as fluids, though antimicrobials may be helpful in preventing acute infections from becoming chronic and in the infrequent cases of septicemia.
Therapy of subacute clinical mastitis: Most cases of clinical mastitis fall into this category. This form of udder inflammation includes only minor alterations in milk such as clots and flakes, or a discolored or watery appearance. The affected quarter occasionally may be slightly swollen and sensitive to the touch with little or no local heat or redness. Although there is often a reduction in milk yield, there are no systemic signs of disease. It is often adequate to perform an intramammary infusion with a Food and Drug Administration-approved lactating cow product accompanied by frequent hand stripping to remove abnormal secretions, bacteria, and cellular debris. Treatment should be continued for at least 24 hours after the disappearance of clinical symptoms under the supervision of the herd veterinarian; otherwise, the infection may only be suppressed to the subclinical level.
A true cure, in which all infecting microorganisms are eliminated from the affected quarter, occurs in less than 50% of cases for most bacterial species. The cure rate is dependent on how long the infection has been present, the age of the cow, the type of organism involved, and other factors such as the SCC at the time that therapy is initiated. Costs associated with treatment of clinical cases include the cost of discarded milk, cost of the drug,and veterinary fees. However, the economic benefits of treatment are less easily recognized because the effect on increasing future milk production, reduction in spread of infections within the herd, decreased chronicity of infection, and decreased culling rates are not obvious.
Therapy of subclinical mastitis: This form of mastitis is the most prevalent type of intramammary infection, but it cannot be detected by looking at the mammary gland or the milk because both appear normal. The majority of infections are caused by the staphylococci and streptococci. If antibiotic therapy is to make a significant contribution toward reducing the herd level of mastitis as well as the bulk tank SCC, it is necessary to treat subclinical infections as well as the clinical cases. It is not unusual to have 15 to 40 subclinical cases for every clinical case caused by contagious pathogens. Generally, intramammary therapy (infusion of mastitis tubes) of subclinical mastitis during lactation is indicated only when Strep. agalactiae is present, or the producer is in danger of losing his milk market due to a high bulk tank SCC.
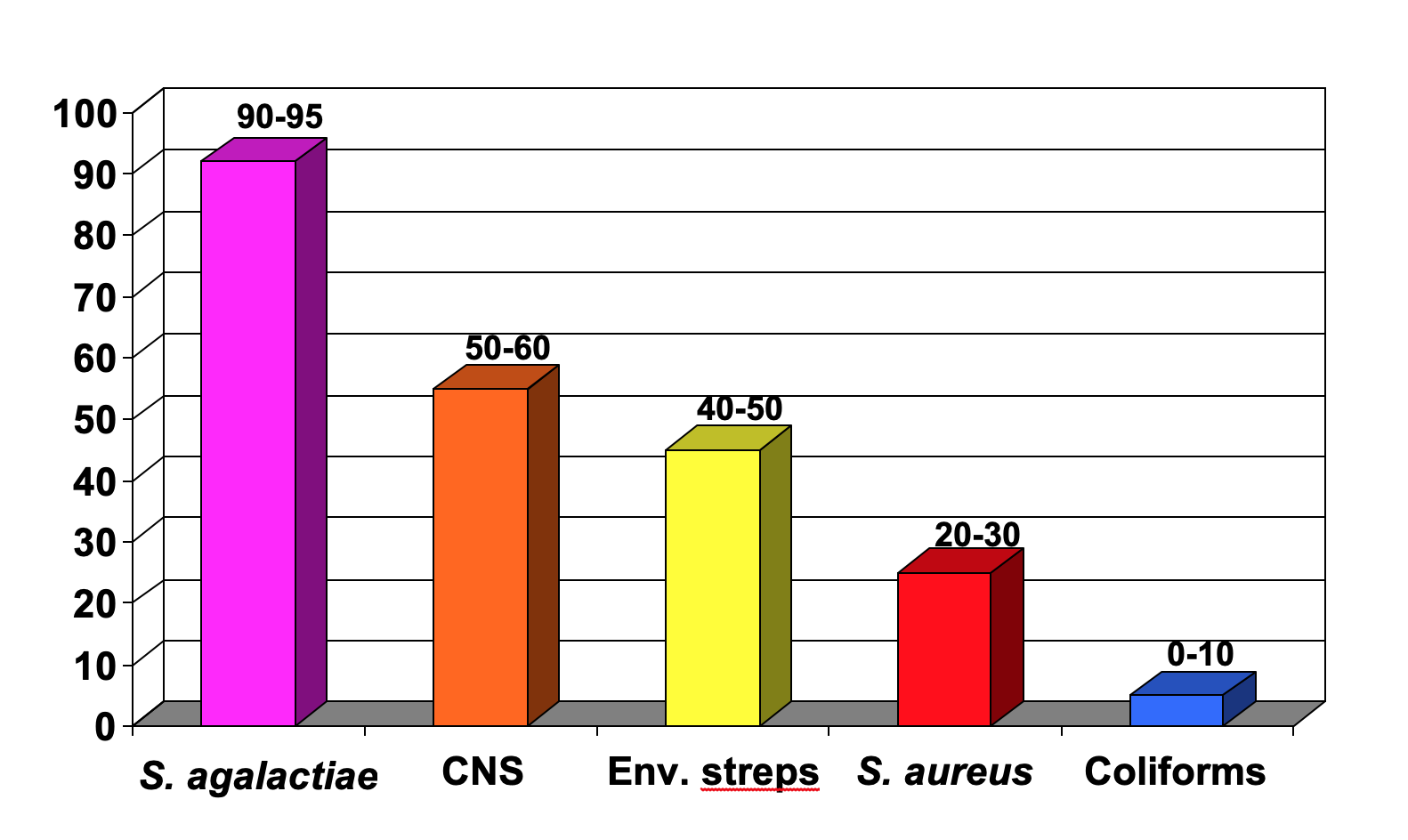
Figure 1 shows the expected cure rates of following antibiotic label instructions for the common mastitis pathogens. Cure rates for Strep. agalactiae are usually in the range of 90-95%. Cure rates of infections caused by other microorganisms during lactation following antibiotic label instructions will be approximately as follows. Coagulase-negative staphylococci (CNS): 50-60%; environmental streptococci (Streptococcus uberis and Streptococcus dysgalactiae): 40-50%; S. aureus: 20-30%; and coliforms: 0-10%. Mycoplasma species, yeasts, Prototheca species, and Nocardia species do not respond to antibiotic therapy. Thus, the organism that is most likely to respond to lactation treatment is Strep. agalactiae. The conventional treatment of other subclinical infections in lactation, such as the CNS, environmental streptococci, S. aureus, and the coliforms is generally not recommended because the cure rate may be as low as 10% and rarely exceeds 50%. Such infections are best treated at drying off or by using the alternative treatment strategies discussed below.
Therapy at drying off
When all quarters of all cows are not treated at drying off, 8-12% of quarters will likely develop a new infection during the dry period, which will be diagnosed at calving. The prevention of only 1% of quarters from becoming infected will pay for the entire dry cow treatment program. Indeed, in herds with a low level of mastitis, preventing new infections during the dry period is more important than curing existing infections. This is because an infected quarter treated at drying off and cured at calving will produce 90% of its potential milk production during the next lactation. However, a quarter that becomes infected during the dry period, or that remains infected from the previous lactation, will produce 30-40% less milk.
Treatment of all quarters of all cows at drying off (blanket dry cow therapy) is one of the most important components of a comprehensive plan of mastitis control. This is because dry cow therapy both cures existing infections, caused mainly by contagious pathogens, and prevents the development of new infections, caused mainly by environmental pathogens. Other advantages of dry cow therapy include the following:
- The cure rate is higher than that experienced during lactation through the use of slow-release products that maintain effective levels of antibiotics for long periods in the nonlactating udder.
- Incidence of new infections during the dry period is reduced.
- Damaged milk-producing tissue is allowed to redevelop before freshening.
- Clinical mastitis at freshening is reduced.
- Salable milk is not contaminated with drug residues.
- All potentially infected quarters are treated with antibiotics.
- Laboratory or screening tests are not required.
To reduce antibiotic usage, selective antibiotic dry cow therapy has become popular in herds with a very low level of contagious pathogens. Selective therapy is based on selecting only the infected or potentially infected quarters (or cows) to treat. This has been promoted to reduce antibiotic expense, drug use, and the development of antibiotic-resistant bacterial strains. The “selection” is based on culture of milk samples and/or use of SCC. For the SCC, a reasonable threshold above which to treat is 200,000/ml, and for cows/quarters assumed to be uninfected, e.g., <200,000/ml, internal teat sealants can be used to prevent new infections.
Studies have demonstrated that in low-SCC cows (<200,000/ml at time of dry-off), selectively treating only those cows diagnosed with udder infections (positive bacterial culture) with both dry cow therapy and an internal teat sealant was as good as blanket dry cow therapy with both products. Other studies have shown that the use of an internal teat sealant alone in low-SCC cows (<200,000/ml at time of dry-off) was as good as dry cow therapy against most bacterial infections, but this method is actually better in preventing coliform infections at the time of calving.
A recent summary of 12 trials evaluating internal teat sealants and dry cow therapy showed that the use of both products in combination or a teat sealant alone were equally effective in reducing the number of new infections as well as clinical mastitis cases at calving. However, the addition of the dry cow therapy lowered SCC at calving, suggesting that the antibiotic component was effective in curing existing infections at the time of drying off, lowering the SCC.
Treatment Procedures
The infusion procedures used to administer intramammary treatments are critical to achieving desired results. In many cases, treatment is administered immediately after milking units are removed, because the udder and teats appear clean due to premilking hygiene as well as the cleansing action of the milking machine. However, the teat end is always contaminated with mastitis-causing bacteria after milking and must be sanitized prior to infusion to minimize the number of bacteria present at the teat that could be carried into the teat canal along with the infusion cannula. These organisms include E. coli, Pseudomonas spp., Prototheca spp., Truperella pyogenes, Nocardia spp., and yeasts. Importantly, many of the aforementioned pathogens are refractory to antibiotics and may render a quarter nonfunctional in severe cases, highlighting the importance of avoiding introduction of these pathogens into the teat,
To minimize microbial contamination, the teat end must be scrubbed vigorously with a 70% alcohol pledget, usually supplied with commercial mastitis tubes, or with cotton balls soaked in 70% alcohol. Teat ends should be cleansed until the pledgets or cotton balls appear unsoiled after the last scrub. In addition, the teat end should be allowed to dry before treatment is administered. It is recommended that gloves be worn when treating cows, especially if highly contagious microorganisms, such as Strep. agalactiae or Mycoplasma bovis, are present in the herd. In addition, hands should be dipped in a sanitizing solution between treating individual animals to reduce spread of pathogens.
The method of drug infusion can actually cause mastitis by inadvertently introducing the microorganisms that are listed in the paragraph above through the teat canal. Full insertion of the conventional mastitis tube syringe cannula can result in temporary dilation of the teat sphincter muscle. In addition, the keratin plug that normally occludes the teat canal is either pushed aside or partially removed. Both of these situations create a larger- than-normal teat canal opening, allowing the entry of microorganisms. The syringe cannula may also push microorganisms that are colonized in keratin into the teat cistern. If the microorganisms gaining access to the teat cistern by these routes are resistant to the infused antibiotic, and most microorganisms are indeed resistant, then a new infection can result. In many instances, the new infection is more severe than the one for which treatment was intended.
Studies designed to compare conventional full insertion with partial insertion of only the first 1/8 inch (2 to 3 millimeters) of the cannula tip have demonstrated that new mastitis cases are minimized by using the partial insertion technique (Figure 2). Several types of syringe cannulas have been developed to aid in inserting only the tip of the cannula into the teat canal. Basically, these partial insertion cannulas are designed to form a seal against the teat opening to provide support during infusion. The partial insertion technique may reduce new infections at calving by 50% or more.
Alternative Treatment Strategies
The purpose of intramammary therapy following label instructions is to help the cow’s natural immune defenses eliminate invading microorganisms. However, the lack of success in curing chronic intramammary infections, particularly those caused by S. aureus and the environmental streps (S. uberis and S. dysgalactiae) has prompted a reevaluation of treatment strategies. Such infections are frequently refractory to conventional intramammary therapy because locally infused antibiotics are not present at high enough concentrations for a sufficient length of time to be effective in killing all the bacteria in the affected quarter.
In addition, the presence of scar tissue, mammary tissue swelling, and milk ducts blockages, all of which may occur in response to S. aureus mastitis, renders the bacteria inaccessible to the infused drug. Thus, these microbes continue to multiply in the milk-producing tissues of the gland. Various treatment methods have been researched in attempts to increase cure rates against mastitis-causing bacteria. These include such procedures as 1) extended therapy in which infected quarters are treated with antibiotics for a longer period of time, and 2) combination therapy in which cows are treated simultaneously in the udder as well as systemically with compatible drugs. Each of these alternative treatment regimens are discussed below.
Extended therapy: This procedure involves the on-label use of a relatively new mastitis drug, Pirsue® (pirlimycin hydrochloride), and is infused over an extended period of time. For the conventional dosage, the product label specifies the user to infuse one syringe into each affected quarter and to repeat treatment after24 hours. For extended therapy, this daily treatment may be repeated at 24-hour intervals for up to eight consecutive days. The milk discard time is 36 hours after the last treatment.
An earlier treatment protocol using three series of on-label treatments of Pirsue® (two infusions 24 hours apart), separated by 36-hour milk discard periods, was evaluated in a commercial dairy herd having issues with S. aureus mastitis. The average cure rate against this organism was 86% of quarters, and SCC in the infected quarters that cured decreased from 3,400,000 to 280,000/ml. Explanations for the high cure rates of pirlimycin include the following: 1) this drug has excellent antimicrobial activity against S. aureus; 2) it penetrates scar tissue quite well; and 3) 50% of the drug is absorbed from the udder into the bloodstream and 50% of that amount is then re-excreted back into the udder, thus aiding the drug in reaching additional infected tissues.
Spectramast® LC can also be used on-label as extended therapy by using a once-a-day intramammary treatment for up to eight straight days; milk withdrawal is 72 hours following last treatment. It offers a broad-spectrum coverage against environmental pathogens.
Extended therapy may also be used off-label using other lactating cow products under the supervision of the herd veterinarian. A 10-year research trial at the UGA Teaching Dairy evaluated Hetacin-K® (PolyMastTM), ToDAY®, Amoxi-Mast®, Pirsue®, and Spectramast® LC under two treatment regimens: 1) following label instructions for each product or 2) as extended therapy. For all drugs evaluated, extended therapy entailed administering one intramammary infusion at each of six consecutive milkings, for a total of six infusions. The various therapies and regimens were used in attempts to cure infections caused by S. aureus, the environmental streptococci, and the coagulase-negative staphylococci (CNS).
The results, which included 125 quarters of 87 lactating cows, was recently summarized and showed that overall cure rate across all five products tested and two treatment regimens for all types of mastitis (S. aureus, environmental streptococci, and CNS) was 58.9%. No differences in cure rates were observed across antibiotic treatment regimens, for example, between using label instructions (57.4% cure) or extended therapy (60.3%). Across treatment products and treatment regimens, cure rates were highest for the CNS (83.9%), followed by the environmental streptococci (59.2%), and S. aureus (50.3%).
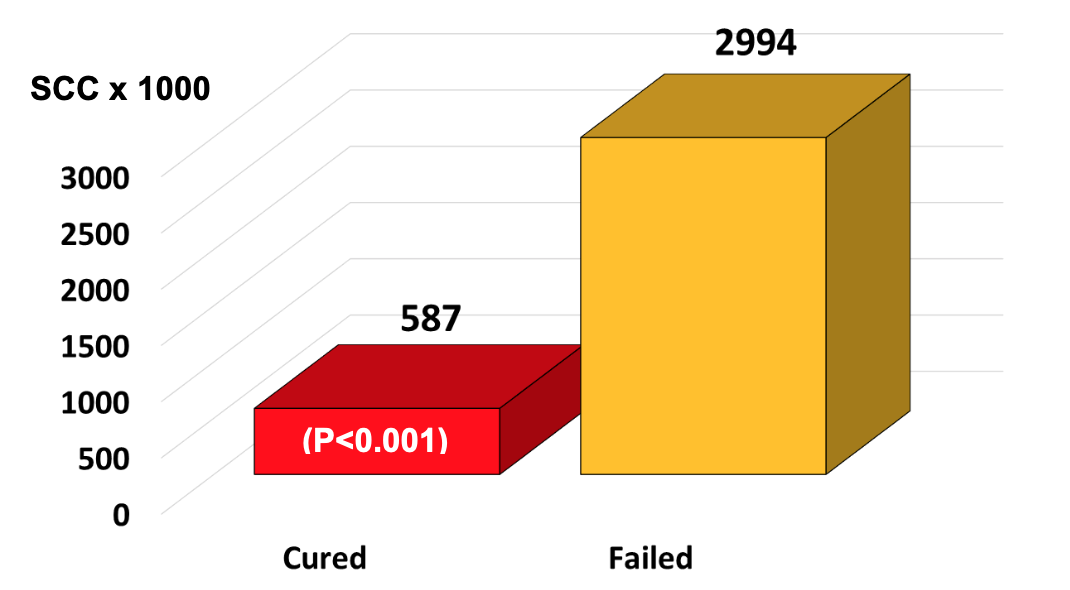
It was very revealing that infected quarters that actually cured as a result of any antibiotic therapy had relatively low initial SCC at the time the antibiotic therapy was initiated. On the other hand, infected quarters having very high initial SCC were, in fact, treatment failures. For example, the average SCC at the time of treatment in any infected quarter destined to cure after therapy was 587,000/ml, whereas the average SCC of quarters destined to fail at the time of treatment was 2,994,000/ml (Figure 3).
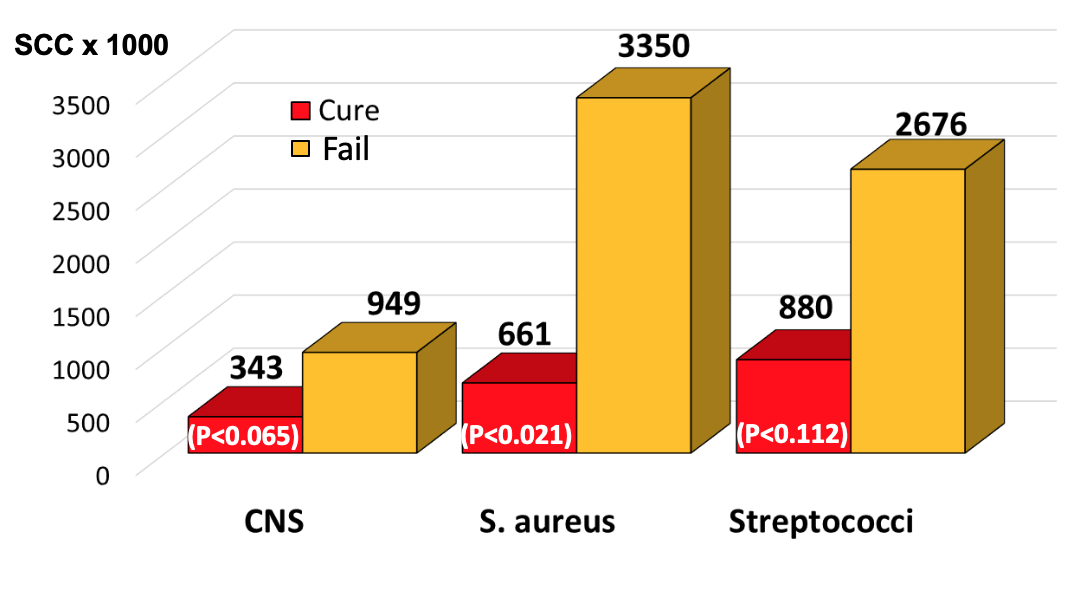
Looking at individual bacterial infections (Figure 4), average SCC at the time of treatment in quarters infected with CNS that were destined to cure after therapy was 343,000/ml, and the mean of those destined to fail was 949,000/ml. The average SCC at the time of treatment in quarters infected with S. aureus that were destined to cure after therapy was 661,000/ml, and the mean of those destined to fail was 3,350,000/ml. The average SCC at the time of treatment in quarters infected with environmental streptococci that were destined to cure after therapy was 880,000/ml, and the mean of those destined to fail was 2,676,000/ml.
From a practical standpoint, the most important finding of this trial to date is that infected quarters that cured had lower SCC (587,000/ml) vs. those that failed (2,994,000/ml). Although results are preliminary, we believe that dairy farmers may be able to use these SCC as bench marks when deciding if an infected quarter should be treated. For example, if the SCC is 500,000 ± 200/ml, then the chances for a cure are good, but if the SCC is 3,000,000 ± 1,000,000/ml, then the chances of curing are poor, and treatment would not be advised.
Combination therapy: Research has demonstrated that the combination of intramuscular injections together with intramammary infusions results in much higher antibiotic concentrations in infected mammary tissues and may result in a higher cure rate than intramuscular injections or intramammary infusions alone. In one university study, combination therapy (intramuscular injections plus intramammary infusions) was more effective in curing chronic S. aureus infections than intramammary infusion alone. In this study, 49 cows with 78 subclinically infected quarters with S. aureus were treated. One group of Jersey cows received intramammary infusion at each milking for six milkings with a lactating cow product containing 62.5 milligrams (mg) amoxicillin (Amoxi- Mast®). Another group of cows received the same intramammary infusion regimen plus intramuscular injections of 6 million units of procaine penicillin G after each milking for three days.
The combination of intramammary and intramuscular treatment cured 51% of quarters (48% of cows) compared with 25% of quarters (30% of cows) for intramammary infusion alone. Thus, combination therapy was about twice as effective as conventional infusion alone. By eight days after treatment, SCC for cured quarters was 340,000/ml compared with 1,900,000/ml for failed quarters. At 21 days after treatment, SCC were 224,000/ml for cured quarters and 1,975,000/ml for failed quarters.
Supportive Therapy and Nursing Care
There is no substitute for good nursing care in promoting animal well-being and hastening recovery from disease, especially when dealing with clinical mastitis. The provision of fresh drinking water, high quality hay, and a comfortable, well ventilated, clean, and dry environment is very important. Also, frequent hand stripping of affected quarters aids in removing toxic substances resulting from infection. Use of the milk let-down hormone, oxytocin, facilitates complete removal of milk, debris, and toxins, will be discussed later. In instances where gangrene develops, surgical removal of the teat by a veterinarian may facilitate drainage of toxic materials and increase the chances of salvaging the cow. If corticosteroids are used as adjunct therapy, the chances of a subsequent bacteremia are increased; therefore, extreme caution in the use of these compounds should be exercised.
Some cows may become hypocalcemic during the course of clinical mastitis. Careful administration of calcium solutions may be undertaken if serum biochemistry values warrant such therapeutic measures. These cows are very susceptible to cardiac arrhythmias, cardiac failure, and death if calcium is administered too rapidly or when not indicated. Daily production, appearance of milk, and water and feed intake should be monitored and recorded to assess the cow’s progress. Cows with severe clinical cases will experience additional undesirable stress, if for example, temperature extremes exist in the hospital environment. Thus, cow comfort is of utmost importance.
Oxytocin Therapy
Oxytocin also has been used to treat clinical forms of mastitis and is also known as the milk let-down hormone. Oxytocin is naturally released from the brain into the bloodstream in response to the stimuli associated with milking. It causes contraction of the milk-producing cells or alveoli within the mammary gland, resulting in the expulsion of milk. It is believed that administrating exogenous (injectable) oxytocin further “squeezes out” or flushes residual bacteria-laden fluid after milking, which aids in eliminating the infection. In addition, inflammatory by-products and bacterial toxins are removed.
Commonly, veterinarians recommend frequent stripping of affected quarters (six times a day) following oxytocin administration (40 to 50 units intramuscularly). This may be accompanied by supportive therapy using aspirin or banamine. Cows usually recover from clinical symptoms within 24 to 48 hours. In one study, oxytocin was injected intramuscularly at 100 units every 12 hours for two or three milkings in attempts to treat clinical mastitis cases. The clinical cure rate (return of the quarter and the milk to normal by 20 days) was about 70%, but the bacteriologic cure rate (absence of the infecting microorganism by day 20) was 49%. This cure rate is similar to that observed after treatment with most antibiotics; however, use of oxytocin does not result in the potential contamination of milk with antibiotic residues, has no withdrawal time, and is less costly. However, as discussed below, relapses can occur. Moreover, in some instances animals may become used to the administration of oxytocin such that their own biologic oxytocin is less effective, causing issues with milk letdown after the mastitis oxytocin regimen is completed.
Veterinarians in California compared the economic benefits of three different treatment regimens, one of which included oxytocin. Cows with mild clinical mastitis were assigned to one of three treatment groups: 1) treatment with 62.5 mg of intramammary amoxicillin every 12 hours for three milkings; 2) treatment with 200 mg of intramammary cephapirin every 12 hours for two milkings; and 3) treatment intramuscularly with 100 units of oxytocin every 12 hours for three milkings. Clinical cure rates were similar among the three groups of animals. However, there was not an economic advantage to oxytocin treatment due to the longer time required for milk from animals in this group to return to normal, with a lack of clots and flakes. In addition, there was a higher relapse rate among the oxytocin-treated cows, and about 65% of them experienced at least one additional case of clinical mastitis during the remainder of lactation. Many of the relapses and additional clinical cases were caused by environmental streptococci. Oxytocin treatment may be cost effective for herds in which coliforms cause a majority of clinical cases and in which environmental streptococci are a minor problem.
Somatic Cell Response to Therapy
A true cure is the elimination of all microorganisms from a previously infected quarter for at least a three-week period after treatments have been terminated. The time required after successful treatment for SCC to decrease substantially depends on the degree of inflammation and amount of tissue damage that resulted from the infection. The range will vary from a few days for microorganisms such as Strep. agalactiae to a few months or even to the next lactation for some S. aureusinfections. Some quarters will be permanently damaged as a result of infection and will produce milk with an elevated SCC indefinitely, and culling may be the best option for such cases.
Conclusions
Conventional lactating cow therapy has generally been less than successful in the treatment of the majority of mastitis-causing bacteria with the exception of Strep. agalactiae. Cure rates for chronic S. aureus mastitis may be improved using extended therapy as well as by the combination of parenteral injections coupled with intramammary infusion of antibiotics. However, in most cases, both approaches require vigilant monitoring of antibiotic residues in milk from treated animals and supervision of the herd veterinarian. For lactating cows, a major factor that may play a role in whether an infected quarter cures or fails is the SCC at the time that treatment is initiated; the lower the SCC the greater the chances for a successful cure. Success of antibiotic treatment against all mastitis-causing bacteria is always greatest when using nonlactating cow therapy administered at the end of lactation, and cure rates against S. aureus approach 75%. More recently, selective antibiotic dry cow therapy (based on selecting only the infected or potentially infected quarters to treat) has become popular in herds and cows with SCC of less than 200,000/ml and a low level of contagious pathogens.








